Nickel »
PDB 4ofo-4rro »
4qwn »
Nickel in PDB 4qwn: Histone Demethylase KDM2A-H3K36ME1-Alpha-Kg Complex Structure
Enzymatic activity of Histone Demethylase KDM2A-H3K36ME1-Alpha-Kg Complex Structure
All present enzymatic activity of Histone Demethylase KDM2A-H3K36ME1-Alpha-Kg Complex Structure:
1.14.11.27;
1.14.11.27;
Protein crystallography data
The structure of Histone Demethylase KDM2A-H3K36ME1-Alpha-Kg Complex Structure, PDB code: 4qwn
was solved by
Z.J.Cheng,
D.J.Patel,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 85.84 / 2.10 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 54.343, 87.568, 171.681, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 21.5 / 26.4 |
Nickel Binding Sites:
The binding sites of Nickel atom in the Histone Demethylase KDM2A-H3K36ME1-Alpha-Kg Complex Structure
(pdb code 4qwn). This binding sites where shown within
5.0 Angstroms radius around Nickel atom.
In total 2 binding sites of Nickel where determined in the Histone Demethylase KDM2A-H3K36ME1-Alpha-Kg Complex Structure, PDB code: 4qwn:
Jump to Nickel binding site number: 1; 2;
In total 2 binding sites of Nickel where determined in the Histone Demethylase KDM2A-H3K36ME1-Alpha-Kg Complex Structure, PDB code: 4qwn:
Jump to Nickel binding site number: 1; 2;
Nickel binding site 1 out of 2 in 4qwn
Go back to
Nickel binding site 1 out
of 2 in the Histone Demethylase KDM2A-H3K36ME1-Alpha-Kg Complex Structure
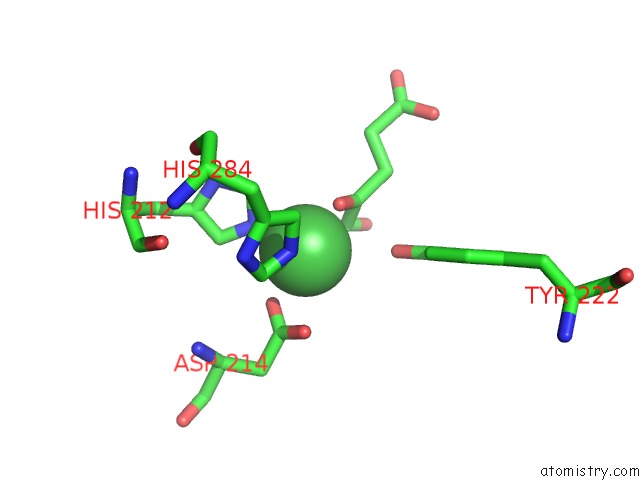
Mono view
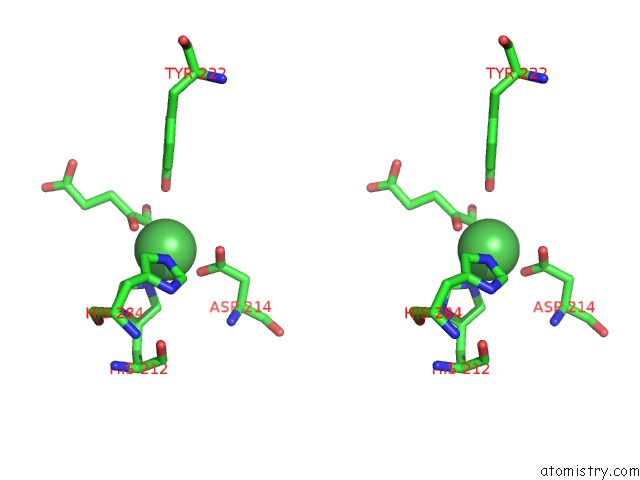
Stereo pair view

Mono view

Stereo pair view
A full contact list of Nickel with other atoms in the Ni binding
site number 1 of Histone Demethylase KDM2A-H3K36ME1-Alpha-Kg Complex Structure within 5.0Å range:
|
Nickel binding site 2 out of 2 in 4qwn
Go back to
Nickel binding site 2 out
of 2 in the Histone Demethylase KDM2A-H3K36ME1-Alpha-Kg Complex Structure
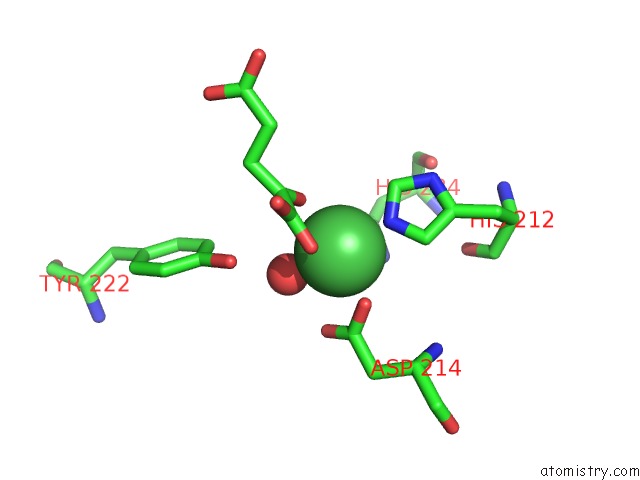
Mono view
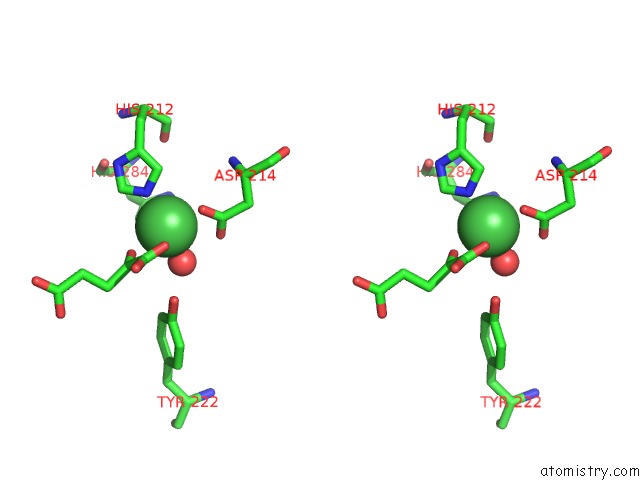
Stereo pair view

Mono view

Stereo pair view
A full contact list of Nickel with other atoms in the Ni binding
site number 2 of Histone Demethylase KDM2A-H3K36ME1-Alpha-Kg Complex Structure within 5.0Å range:
|
Reference:
Z.Cheng,
P.Cheung,
A.J.Kuo,
E.T.Yukl,
C.M.Wilmot,
O.Gozani,
D.J.Patel.
A Molecular Threading Mechanism Underlies Jumonji Lysine Demethylase KDM2A Regulation of Methylated H3K36. Genes Dev. V. 28 1758 2014.
ISSN: ISSN 0890-9369
PubMed: 25128496
DOI: 10.1101/GAD.246561.114
Page generated: Mon Aug 18 19:38:14 2025
ISSN: ISSN 0890-9369
PubMed: 25128496
DOI: 10.1101/GAD.246561.114
Last articles
K in 9NESK in 9PHG
K in 9NEI
K in 9NED
K in 9NEC
K in 9NEG
K in 9CWU
K in 9CVB
K in 9CVA
K in 9COM