Nickel »
PDB 1fwc-1j6p »
1hbu »
Nickel in PDB 1hbu: Methyl-Coenzyme M Reductase in the Mcr-RED1-Silent State in Complex with Coenzyme M
Protein crystallography data
The structure of Methyl-Coenzyme M Reductase in the Mcr-RED1-Silent State in Complex with Coenzyme M, PDB code: 1hbu
was solved by
U.Ermler,
W.Grabarse,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 10.00 / 1.90 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 81.100, 116.500, 121.800, 90.00, 91.80, 90.00 |
| R / Rfree (%) | 16.2 / 21.1 |
Other elements in 1hbu:
The structure of Methyl-Coenzyme M Reductase in the Mcr-RED1-Silent State in Complex with Coenzyme M also contains other interesting chemical elements:
| Magnesium | (Mg) | 13 atoms |
| Zinc | (Zn) | 1 atom |
| Chlorine | (Cl) | 2 atoms |
| Sodium | (Na) | 9 atoms |
Nickel Binding Sites:
The binding sites of Nickel atom in the Methyl-Coenzyme M Reductase in the Mcr-RED1-Silent State in Complex with Coenzyme M
(pdb code 1hbu). This binding sites where shown within
5.0 Angstroms radius around Nickel atom.
In total 2 binding sites of Nickel where determined in the Methyl-Coenzyme M Reductase in the Mcr-RED1-Silent State in Complex with Coenzyme M, PDB code: 1hbu:
Jump to Nickel binding site number: 1; 2;
In total 2 binding sites of Nickel where determined in the Methyl-Coenzyme M Reductase in the Mcr-RED1-Silent State in Complex with Coenzyme M, PDB code: 1hbu:
Jump to Nickel binding site number: 1; 2;
Nickel binding site 1 out of 2 in 1hbu
Go back to
Nickel binding site 1 out
of 2 in the Methyl-Coenzyme M Reductase in the Mcr-RED1-Silent State in Complex with Coenzyme M
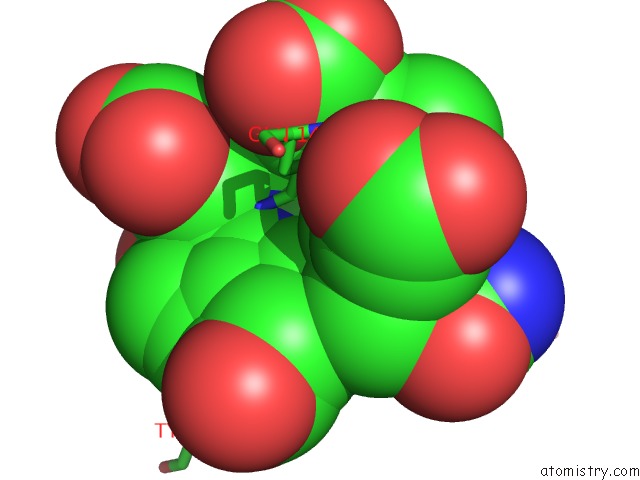
Mono view
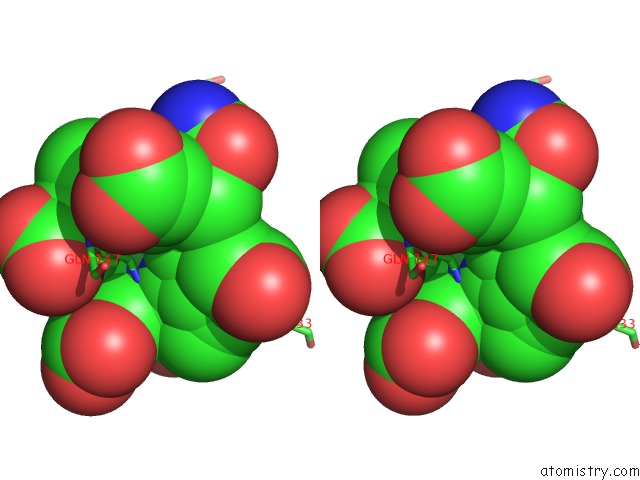
Stereo pair view

Mono view

Stereo pair view
A full contact list of Nickel with other atoms in the Ni binding
site number 1 of Methyl-Coenzyme M Reductase in the Mcr-RED1-Silent State in Complex with Coenzyme M within 5.0Å range:
|
Nickel binding site 2 out of 2 in 1hbu
Go back to
Nickel binding site 2 out
of 2 in the Methyl-Coenzyme M Reductase in the Mcr-RED1-Silent State in Complex with Coenzyme M
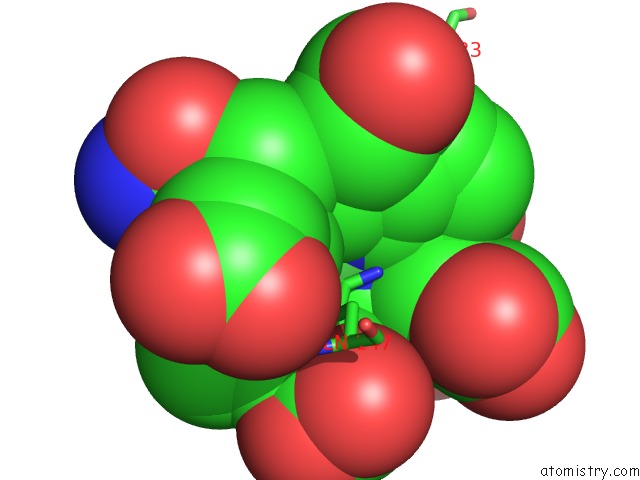
Mono view
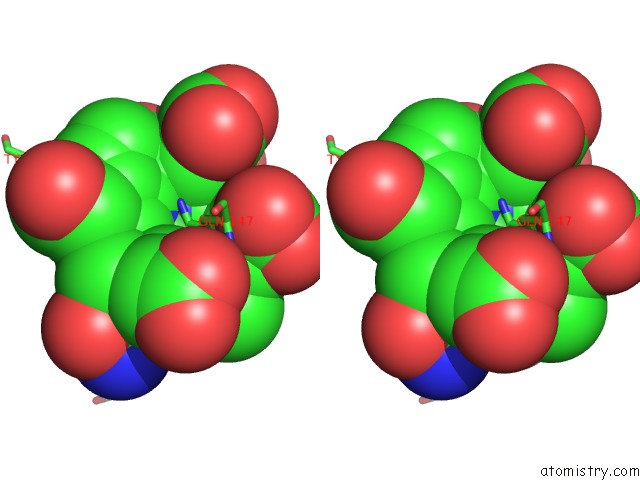
Stereo pair view

Mono view

Stereo pair view
A full contact list of Nickel with other atoms in the Ni binding
site number 2 of Methyl-Coenzyme M Reductase in the Mcr-RED1-Silent State in Complex with Coenzyme M within 5.0Å range:
|
Reference:
W.Grabarse,
F.Mahlert,
E.C.Duin,
M.Goubeaud,
S.Shima,
R.K.Thauer,
V.Lamzin,
U.Ermler.
On the Mechanism of Biological Methane Formation: Structural Evidence For Conformational Changes in Methyl-Coenzyme M Reductase Upon Substrate Binding J.Mol.Biol. V. 309 315 2001.
ISSN: ISSN 0022-2836
PubMed: 11491299
DOI: 10.1006/JMBI.2001.4647
Page generated: Wed Oct 9 14:56:19 2024
ISSN: ISSN 0022-2836
PubMed: 11491299
DOI: 10.1006/JMBI.2001.4647
Last articles
Fe in 3MM3Fe in 3MM2
Fe in 3MM1
Fe in 3ML1
Fe in 3MKB
Fe in 3MJP
Fe in 3MJU
Fe in 3MI5
Fe in 3MJN
Fe in 3MI1