Nickel »
PDB 5bu6-5e6j »
5c74 »
Nickel in PDB 5c74: Structure of A Novel Protein Arginine Methyltransferase
Protein crystallography data
The structure of Structure of A Novel Protein Arginine Methyltransferase, PDB code: 5c74
was solved by
F.Lv,
J.Ding,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 42.16 / 1.90 |
| Space group | P 41 21 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 107.570, 107.570, 87.522, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 19.3 / 23.2 |
Nickel Binding Sites:
The binding sites of Nickel atom in the Structure of A Novel Protein Arginine Methyltransferase
(pdb code 5c74). This binding sites where shown within
5.0 Angstroms radius around Nickel atom.
In total 4 binding sites of Nickel where determined in the Structure of A Novel Protein Arginine Methyltransferase, PDB code: 5c74:
Jump to Nickel binding site number: 1; 2; 3; 4;
In total 4 binding sites of Nickel where determined in the Structure of A Novel Protein Arginine Methyltransferase, PDB code: 5c74:
Jump to Nickel binding site number: 1; 2; 3; 4;
Nickel binding site 1 out of 4 in 5c74
Go back to
Nickel binding site 1 out
of 4 in the Structure of A Novel Protein Arginine Methyltransferase
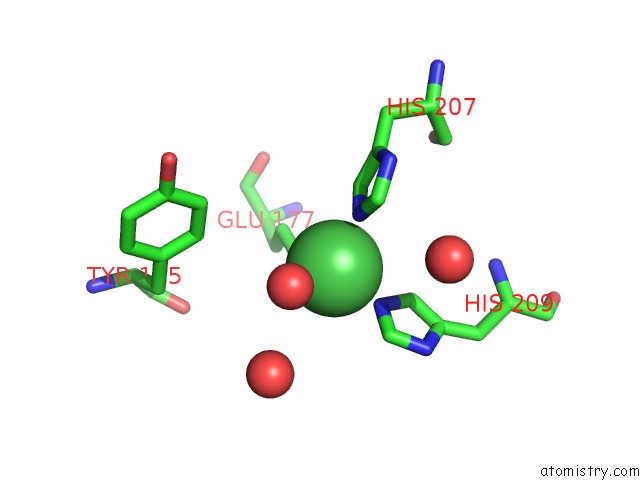
Mono view
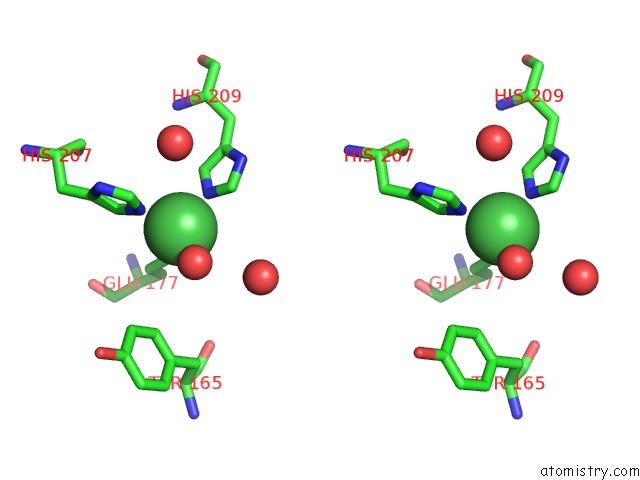
Stereo pair view

Mono view

Stereo pair view
A full contact list of Nickel with other atoms in the Ni binding
site number 1 of Structure of A Novel Protein Arginine Methyltransferase within 5.0Å range:
|
Nickel binding site 2 out of 4 in 5c74
Go back to
Nickel binding site 2 out
of 4 in the Structure of A Novel Protein Arginine Methyltransferase
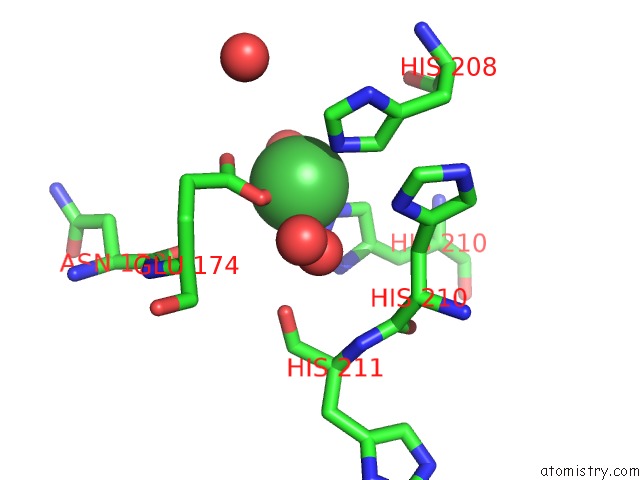
Mono view
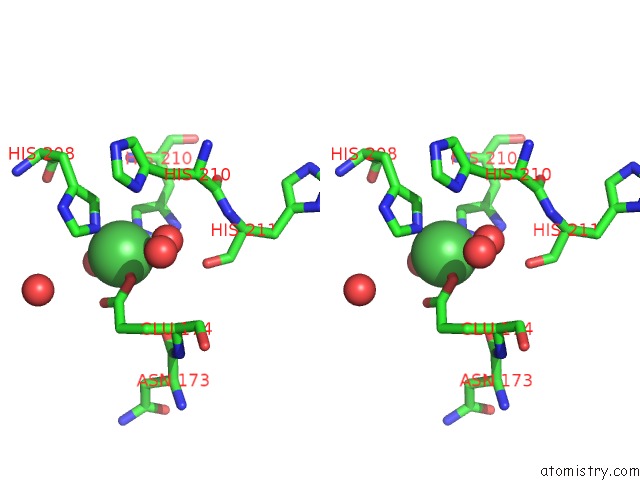
Stereo pair view

Mono view

Stereo pair view
A full contact list of Nickel with other atoms in the Ni binding
site number 2 of Structure of A Novel Protein Arginine Methyltransferase within 5.0Å range:
|
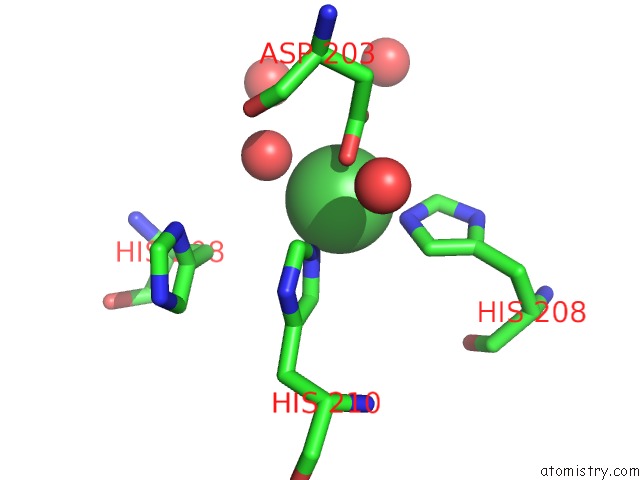
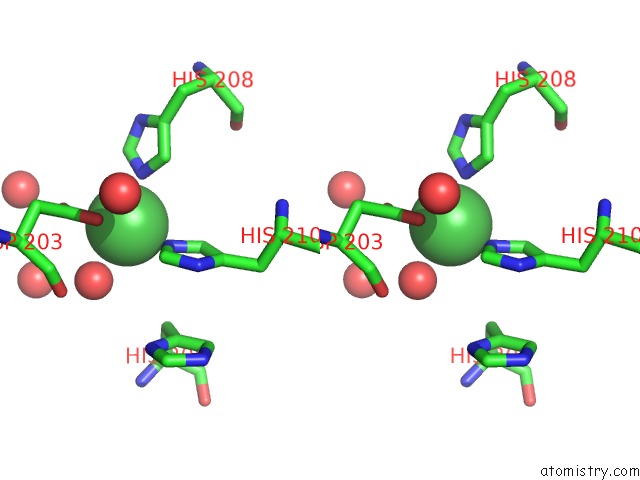
Nickel binding site 3 out of 4 in 5c74
Go back to
Nickel binding site 3 out
of 4 in the Structure of A Novel Protein Arginine Methyltransferase
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Nickel with other atoms in the Ni binding
site number 3 of Structure of A Novel Protein Arginine Methyltransferase within 5.0Å range:
|
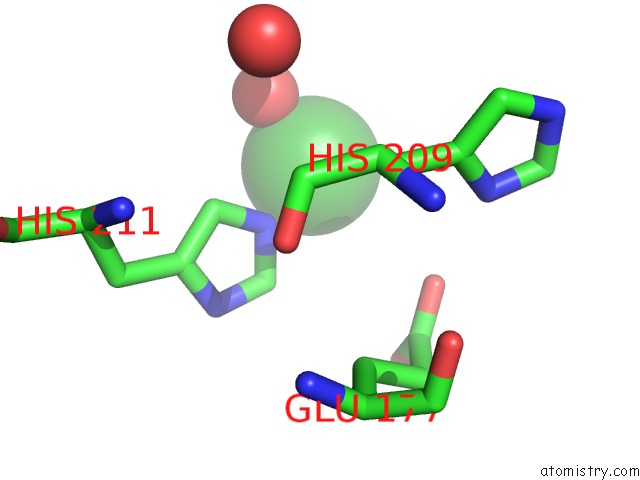
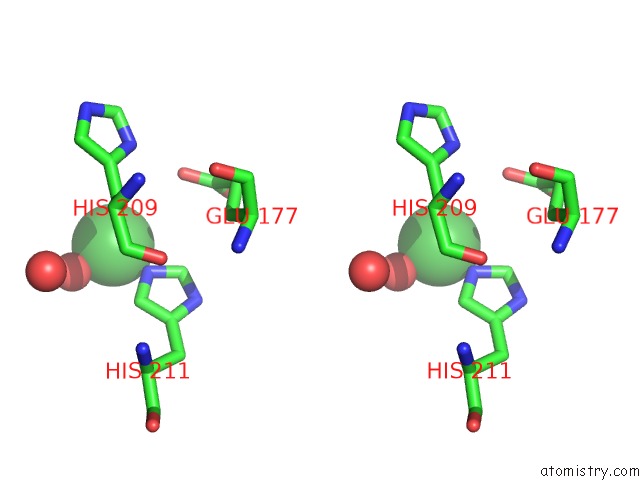
Nickel binding site 4 out of 4 in 5c74
Go back to
Nickel binding site 4 out
of 4 in the Structure of A Novel Protein Arginine Methyltransferase
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Nickel with other atoms in the Ni binding
site number 4 of Structure of A Novel Protein Arginine Methyltransferase within 5.0Å range:
|
Reference:
F.Lv,
T.Zhang,
Z.Zhou,
S.Gao,
C.Wong,
J.Q.Zhou,
J.Ding.
Structural Basis For SFM1 Functioning As A Protein Arginine Methyltransferase Cell Discov 2015.
ISSN: ESSN 2056-5968
DOI: 10.1038/CELLDISC.2015.37
Page generated: Thu Oct 10 06:16:54 2024
ISSN: ESSN 2056-5968
DOI: 10.1038/CELLDISC.2015.37
Last articles
I in 8E7FI in 8DOP
I in 8E5B
I in 8E59
I in 8E58
I in 8E57
I in 8DC1
I in 8E56
I in 8DXL
I in 8DV3