Nickel »
PDB 3hy4-3kvb »
3k0h »
Nickel in PDB 3k0h: The Crystal Structure of BRCA1 Brct in Complex with A Minimal Recognition Tetrapeptide with An Amidated C-Terminus
Protein crystallography data
The structure of The Crystal Structure of BRCA1 Brct in Complex with A Minimal Recognition Tetrapeptide with An Amidated C-Terminus, PDB code: 3k0h
was solved by
S.J.Campbell,
R.A.Edwards,
J.N.Glover,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 29.44 / 2.70 |
| Space group | P 61 2 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 115.075, 115.075, 123.255, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 24.7 / 29.6 |
Other elements in 3k0h:
The structure of The Crystal Structure of BRCA1 Brct in Complex with A Minimal Recognition Tetrapeptide with An Amidated C-Terminus also contains other interesting chemical elements:
| Chlorine | (Cl) | 1 atom |
Nickel Binding Sites:
The binding sites of Nickel atom in the The Crystal Structure of BRCA1 Brct in Complex with A Minimal Recognition Tetrapeptide with An Amidated C-Terminus
(pdb code 3k0h). This binding sites where shown within
5.0 Angstroms radius around Nickel atom.
In total only one binding site of Nickel was determined in the The Crystal Structure of BRCA1 Brct in Complex with A Minimal Recognition Tetrapeptide with An Amidated C-Terminus, PDB code: 3k0h:
In total only one binding site of Nickel was determined in the The Crystal Structure of BRCA1 Brct in Complex with A Minimal Recognition Tetrapeptide with An Amidated C-Terminus, PDB code: 3k0h:
Nickel binding site 1 out of 1 in 3k0h
Go back to
Nickel binding site 1 out
of 1 in the The Crystal Structure of BRCA1 Brct in Complex with A Minimal Recognition Tetrapeptide with An Amidated C-Terminus
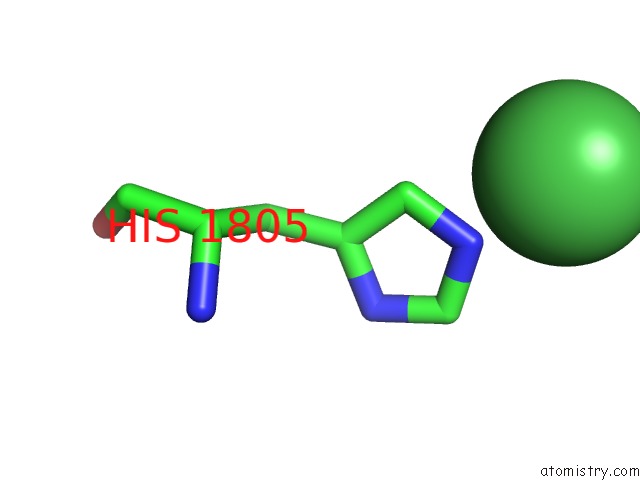
Mono view
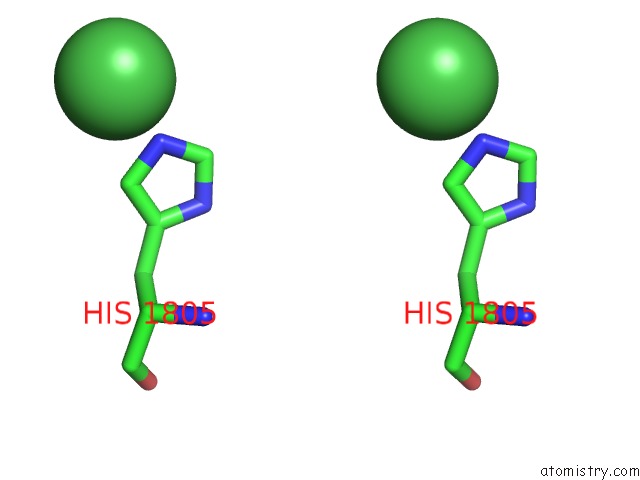
Stereo pair view

Mono view

Stereo pair view
A full contact list of Nickel with other atoms in the Ni binding
site number 1 of The Crystal Structure of BRCA1 Brct in Complex with A Minimal Recognition Tetrapeptide with An Amidated C-Terminus within 5.0Å range:
|
Reference:
S.J.Campbell,
R.A.Edwards,
J.N.Glover.
Comparison of the Structures and Peptide Binding Specificities of the Brct Domains of MDC1 and BRCA1 Structure V. 18 167 2010.
ISSN: ISSN 0969-2126
PubMed: 20159462
DOI: 10.1016/J.STR.2009.12.008
Page generated: Wed Oct 9 17:25:58 2024
ISSN: ISSN 0969-2126
PubMed: 20159462
DOI: 10.1016/J.STR.2009.12.008
Last articles
Al in 3AR8Al in 2YNM
Al in 2ZJY
Al in 2ZBG
Al in 2ZBD
Al in 2Y3I
Al in 2YBE
Al in 2XZO
Al in 2XZL
Al in 2WOJ