Nickel »
PDB 3l1m-3n6n »
3l6t »
Nickel in PDB 3l6t: Crystal Structure of An N-Terminal Mutant of the Plasmid PCU1 Trai Relaxase Domain
Protein crystallography data
The structure of Crystal Structure of An N-Terminal Mutant of the Plasmid PCU1 Trai Relaxase Domain, PDB code: 3l6t
was solved by
M.R.Redinbo,
R.P.Nash,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 31.37 / 1.93 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 50.385, 58.656, 86.532, 90.00, 95.01, 90.00 |
| R / Rfree (%) | 16.8 / 20.4 |
Other elements in 3l6t:
The structure of Crystal Structure of An N-Terminal Mutant of the Plasmid PCU1 Trai Relaxase Domain also contains other interesting chemical elements:
| Magnesium | (Mg) | 1 atom |
| Chlorine | (Cl) | 3 atoms |
| Sodium | (Na) | 1 atom |
Nickel Binding Sites:
The binding sites of Nickel atom in the Crystal Structure of An N-Terminal Mutant of the Plasmid PCU1 Trai Relaxase Domain
(pdb code 3l6t). This binding sites where shown within
5.0 Angstroms radius around Nickel atom.
In total 2 binding sites of Nickel where determined in the Crystal Structure of An N-Terminal Mutant of the Plasmid PCU1 Trai Relaxase Domain, PDB code: 3l6t:
Jump to Nickel binding site number: 1; 2;
In total 2 binding sites of Nickel where determined in the Crystal Structure of An N-Terminal Mutant of the Plasmid PCU1 Trai Relaxase Domain, PDB code: 3l6t:
Jump to Nickel binding site number: 1; 2;
Nickel binding site 1 out of 2 in 3l6t
Go back to
Nickel binding site 1 out
of 2 in the Crystal Structure of An N-Terminal Mutant of the Plasmid PCU1 Trai Relaxase Domain
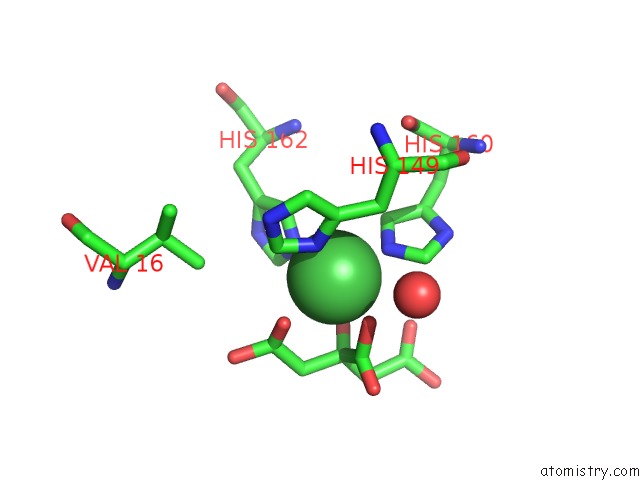
Mono view
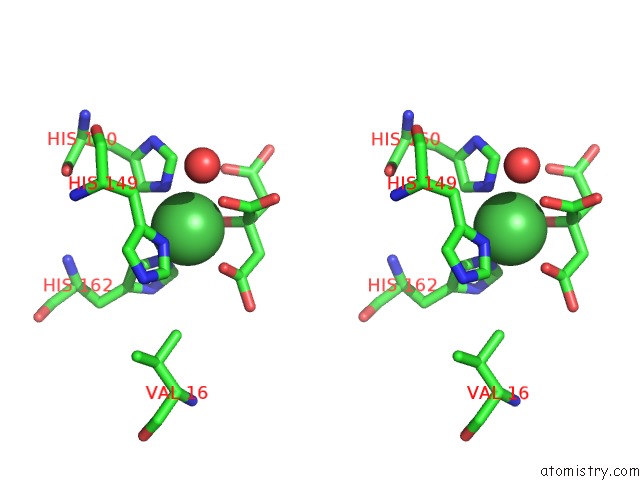
Stereo pair view

Mono view

Stereo pair view
A full contact list of Nickel with other atoms in the Ni binding
site number 1 of Crystal Structure of An N-Terminal Mutant of the Plasmid PCU1 Trai Relaxase Domain within 5.0Å range:
|
Nickel binding site 2 out of 2 in 3l6t
Go back to
Nickel binding site 2 out
of 2 in the Crystal Structure of An N-Terminal Mutant of the Plasmid PCU1 Trai Relaxase Domain
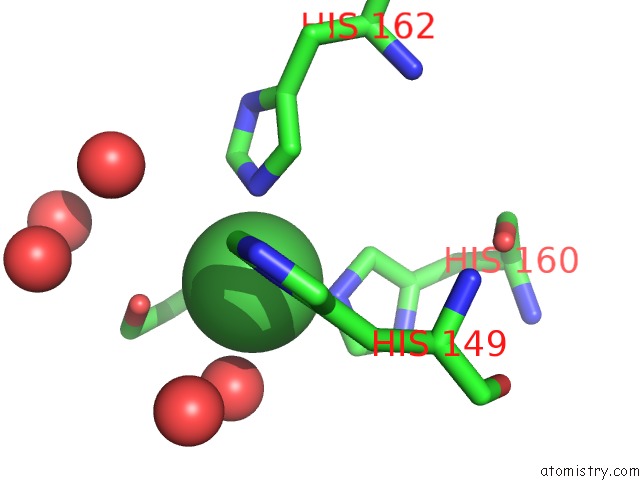
Mono view
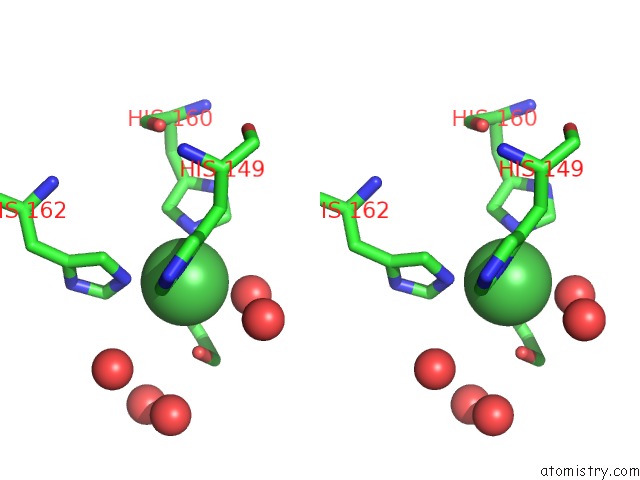
Stereo pair view

Mono view

Stereo pair view
A full contact list of Nickel with other atoms in the Ni binding
site number 2 of Crystal Structure of An N-Terminal Mutant of the Plasmid PCU1 Trai Relaxase Domain within 5.0Å range:
|
Reference:
R.P.Nash,
S.Habibi,
Y.Cheng,
S.A.Lujan,
M.R.Redinbo.
The Mechanism and Control of Dna Transfer By the Conjugative Relaxase of Resistance Plasmid PCU1. Nucleic Acids Res. V. 38 5929 2010.
ISSN: ISSN 0305-1048
PubMed: 20448025
DOI: 10.1093/NAR/GKQ303
Page generated: Mon Aug 18 18:45:05 2025
ISSN: ISSN 0305-1048
PubMed: 20448025
DOI: 10.1093/NAR/GKQ303
Last articles
Ni in 6ETENi in 6ETS
Ni in 6ETG
Ni in 6ELQ
Ni in 6EN9
Ni in 6EOZ
Ni in 6EN3
Ni in 6EI2
Ni in 6EK6
Ni in 6EHS