Nickel »
PDB 3qsi-3tsn »
3rdq »
Nickel in PDB 3rdq: Crystal Structure of R7-2 Streptavidin Complexed with Desthiobiotin
Protein crystallography data
The structure of Crystal Structure of R7-2 Streptavidin Complexed with Desthiobiotin, PDB code: 3rdq
was solved by
V.N.Malashkevich,
M.Magalhaes,
C.M.Czecster,
R.Guan,
M.Levy,
S.C.Almo,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 19.98 / 1.60 |
| Space group | I 41 2 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 57.275, 57.275, 183.742, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 16.1 / 19 |
Other elements in 3rdq:
The structure of Crystal Structure of R7-2 Streptavidin Complexed with Desthiobiotin also contains other interesting chemical elements:
| Sodium | (Na) | 1 atom |
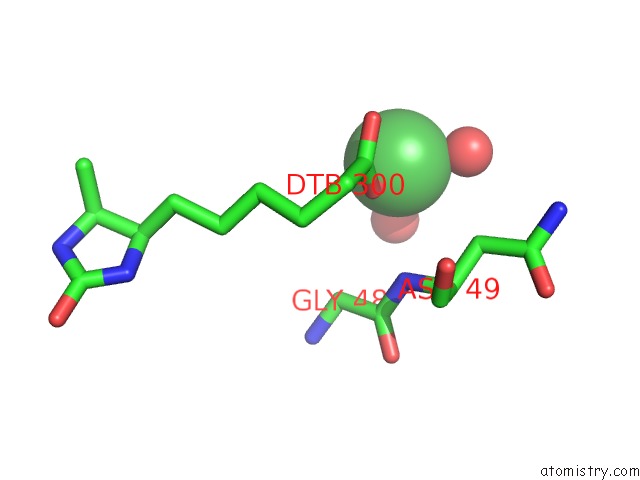
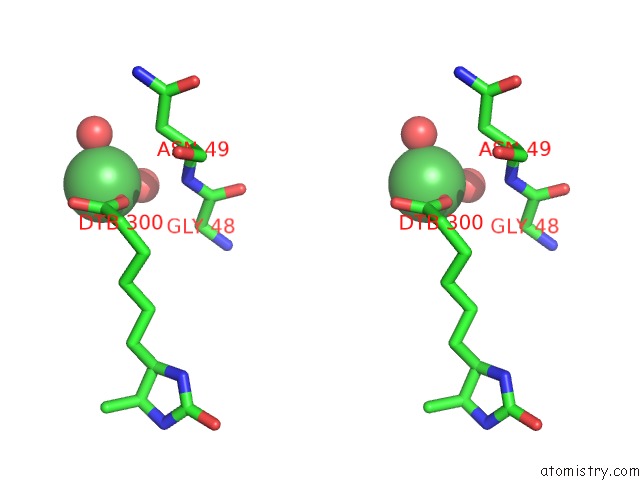
Nickel Binding Sites:
The binding sites of Nickel atom in the Crystal Structure of R7-2 Streptavidin Complexed with Desthiobiotin
(pdb code 3rdq). This binding sites where shown within
5.0 Angstroms radius around Nickel atom.
In total only one binding site of Nickel was determined in the Crystal Structure of R7-2 Streptavidin Complexed with Desthiobiotin, PDB code: 3rdq:
In total only one binding site of Nickel was determined in the Crystal Structure of R7-2 Streptavidin Complexed with Desthiobiotin, PDB code: 3rdq:
Nickel binding site 1 out of 1 in 3rdq
Go back to
Nickel binding site 1 out
of 1 in the Crystal Structure of R7-2 Streptavidin Complexed with Desthiobiotin
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Nickel with other atoms in the Ni binding
site number 1 of Crystal Structure of R7-2 Streptavidin Complexed with Desthiobiotin within 5.0Å range:
|
Reference:
M.L.Magalhaes,
C.M.Czekster,
R.Guan,
V.N.Malashkevich,
S.C.Almo,
M.Levy.
Evolved Streptavidin Mutants Reveal Key Role of Loop Residue in High-Affinity Binding. Protein Sci. V. 20 1145 2011.
ISSN: ISSN 0961-8368
PubMed: 21520321
DOI: 10.1002/PRO.642
Page generated: Mon Aug 18 18:57:14 2025
ISSN: ISSN 0961-8368
PubMed: 21520321
DOI: 10.1002/PRO.642
Last articles
Ni in 5U9ENi in 5TVS
Ni in 5U93
Ni in 5TVR
Ni in 5TRQ
Ni in 5TFZ
Ni in 5TR8
Ni in 5T22
Ni in 5TCW
Ni in 5TBX